Investigations into the Role of Aging in Carcinogenesis:
Focus on
Tumor Progression
Once considered a cell-based process, progression of existing cancers
is now appreciated to involve elaborate tumor-host interactions,
including, but not limited to, angiogenesis, matrix remodeling, and
immune editing. Although the conduciveness of the host to tumor
advancement is itself strongly dependent on host age, the direct
role of age as a modifier of cancer progression remains
unexplored. This is true despite age being among the strongest risk
factors for cancer incidence. Epidemiological data show that from
adolescence through middle age, cancer incidence increases with age,
while during middle-age the incidence begins to decelerate and,
surprisingly, it actually decreases at sufficiently advanced ages,
for many tumor sites (Fig. 1). Several theories have been proposed
to account for the decreased incidence observed at old age
including: 1) Incidence data obtained may not be accurately
collected in older patients; however, this notion is not supported
by studies done on animal models which demonstrate the same pattern
as observed in humans, 2) A widely accepted theory that no doubt
contributes, is that there is heterogeneity among individuals with
respect to intrinsic cancer susceptibility, also referred to as
frailty. More susceptible individuals develop cancer and are removed
from the statistical population leaving, on average, a population of
less susceptible individuals at older ages, and 3)
Age-dependent host influences that act to modify tumor
promotion and/or progression, which now appear, at sufficiently old
ages, to inhibit tumor progression and actually lower clinical
incidence. At CCSB, our research reveals this reduced
capacity of older hosts to support tumor advancement, which offers
insight into an important phenomenon that limits tumor progression
and incidence at advanced age.
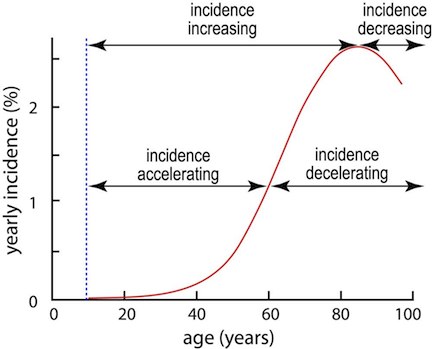 |
Figure 1. Schematic of U.S. cancer incidence based on Surveillance, Epidemiology and End Results (SEER) data for cancers at all sites. The vertical axis is age-specific cancer incidence. The curve indicates, that among 100 individuals aged 70 who have not previously had cancer, about 2 will present with cancer during their 70th year. It is seen that from age ~10 to age ~85 incidence increases (i.e. the slope of the curve is positive); thereafter, as has been recently realized, there is for some sites actually a decrease (negative slope) in cancer incidence at these advanced ages. From age ~10 to age ~60 incidence accelerates (slope is increasing). Around 60yrs of age a maximum slope is reached and thereafter incidence is decelerating, characterized by decreasing slope. Formally, the slope is given by the first derivative of the incidence function; acceleration corresponds to a positive second derivative, and deceleration to a negative second derivative. [Beheshti et al, 2013] |
Host Dependent Changes as a Function of Age Impact Tumor Progression
The literature associates a number of age-related changes with lower
tumor progression. These include loss in host angiogenic potential
altered immune response, and age-related cellular senescence. Although
many of investigations have focused on decreased angiogenic factors
that contribute to slower tumor progression at older ages, they often
fail to consider the simultaneous impact of other systemic factors of
the aged hosts on tumor progression. A more global age related
perspective was recently discussed by López-Otín et al.
[López-Otín et al, 2013],
“hallmarks” of aging were identified, but little was discussed as to how these
hallmarks would impact carcinogenesis other than acknowledging that
aging and cancer can be considered as two conditions deriving from
many of the same processes (i.e. increases in mutations and
accumulating cellular damage). To reveal a more global appreciation of
the impact of aged hosts on carcinogenesis, particularly the tumor
progression phases, we at CCSB utilize a systems biology approach. For
examining progression of tumors as a function of age, in vivo
syngeneic tumor studies coupled with molecular and -omics analysis are
undertaken and the data incorporated into multi-scale mathematical
models.
Our investigations have shown modulation of tumor progression,
and growth as a function of host age, is attributable to specific
genetic and functional changes between the tumor/host that affect the
ability of the host to support that growth. Global gene array analysis
was performed on excised tumors from different host (mice) age groups
injected with the same population of cancer cells. Differential gene
expression observed for tumors from different aged mice is then
directly attributable to differences in host age. Differential gene
regulations among tumors from the various host ages were attributable
to host interactions. Thus revealing crucial genetic regulations and
shedding light on the host functional process impacting the tumor
dynamics observed. Tumor inhibitory effects observed with age were
attributed primarily to classes of genes, including those regulating
metabolism, and transcriptional regulators modulating angiogenesis,
and apoptosis. One key player revealed was Transforming growth
factor, beta 1 (TGFβ1), which, as it turns out, is also found to be
integral to a number of functional “hallmarks of aging” e,g,
intercellular communication, stem cell exhaustion, telomere attrition,
etc. Employing a systems biology approach, the tumor growth dynamics
for cohorts of various aged mice were further linked to the underlying
biology using a version of the mathematical model of Hahnfeldt et
al. [Hahnfeldt et al, 1999] in which the quantiative
construct describing the tumor carrying capacity of the host under angiogenic signaling is
modified to allow for evaluation of the age-dependent variation in
the “carrying capacity”, i.e., the tumor size that could potentially
be supported by the current host microenvironmental state at a given
age. Associating carrying capacity with angiogenesis state, individual
fittings of the tumor growth model revealed substantial differences in
the capacity to support tumor progression with host age, as quantified
by parameter responsiveness to tumor-derived stimulatory and
inhibitory signals. More generally, our studies reveal that aging
itself appears to be a powerful orchestrator of global gene and tissue
function to the specific end that the aging host presents resistance
to the pathological changes characteristic of advancing cancer
disease. The ability of age to exert control over such a panoply of
tumor progression regulators, with the end result being suppression of
disease progression, allows for the construction of such tumor/age
models which offer insights into the modulation of a multitude of
molecular processes that in aggregate offer a means to gain a more
sweeping control of the cancer progression process that has heretofore
defied control at the level of specific genes.
Effects of Age on Tumor Growth and Progression Following Proton Irradiation
Over the past decade, proton therapy has attracted considerable
attention within the radiation oncology community. There are now about
40 proton centers dedicated to treatment of a wide range of
cancers. Accepted advantages demonstrated for proton therapy, over
conventional x-ray radiotherapy, include decreased dosing of normal
tissue, with consequent decreased side effects, and improved targeting
of treatment to tumors within close proximity of vital organs.
Recently in pre-clinical models, proton irradiation has been shown to
modulate several key processes critical in tumor advancement and
progression, including angiogenesis and immunogenicity. In addition to
clinical importance, proton irradiation also needs to be better
understood when dealing with risk of space travel, since a large
component of space radiation derives from protons. At CCSB we examine
the role of interactions between cancer and host cells, and how tumors
develop differently as a function of age and of irradiation. We have
shown that tumor growth is modulated by proton irradiation, with
increased inhibition and a significant radiation-altered molecular
fingerprint evident in tumors grown in old hosts. Through global
transcriptome analysis, TGFβ1 and TGFβ2 were determined to
be key players that contributed to the tumor dynamics observed
(Fig. 2). These findings point to old hosts exhibiting a reduced
capacity to support tumor advancement, which can be further reduced by
proton irradiation.
click image to enlarge in new
window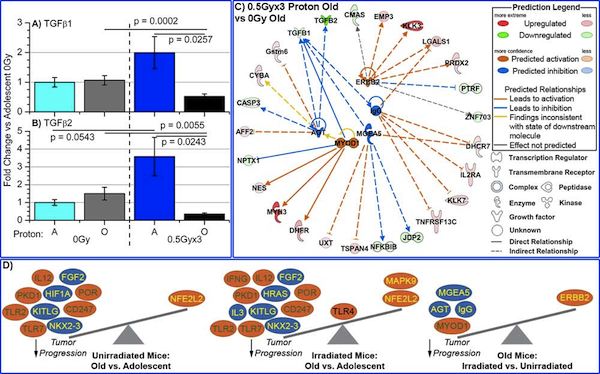 |
Figure 2. Characterization of TGFβ1 and TGFβ2 in tumors from proton irradiated and unirradiated adolescent and old mice. The mRNA expression for A) TGFβ1 and for B) TGFβ2, was determined by real-time PCR (RTPCR). Fold changes for mRNA expression were determined for each group, compared to 0Gy adolescent mice. Significance is indicated by the p-values within the plots. C) Gene network depiction of upstream regulators predicted to be either activated (orange) or inhibited (blue) in tumors from irradiated old mice versus from unirradiated old mice, determined by IPA software. Specific up-regulated (red) and down-regulated (green) genes from the experimental data set involved in determining the activation state of the upstream regulator are shown with direct (—solid lines) and indirect (- - dashed lines) relationships to the upstream regulators. The predicted relationships are color coded to indicate whether it leads to activation (orange) or inhibition (blue). Relationships that are inconsistent with the prediction (yellow) or have an undetermined effect (grey) are also shown. The darker the shade of green or red, the greater the fold change. D) A schematic of the activation states of the upstream regulators illustrating the balance between the tumor promoters (text in yellow) and tumor suppressors (text in green) with a predicted activation (orange oval) or predicted inhibition (blue oval). [Beheshti et al, 2014] |
The Effects of Age on HZE Radiation Response
Since astronaut age is a consideration for extended space
missions, understanding the role of age in modulating spontaneous
and HZE radiation-induced cancer risk is of considerable
interest. Epidemiological studies show that after childhood
spontaneous tumor incidence increases, but in late middle-age
tumor incidence starts to decrease. The increase or decrease of
tumor incidence does not automatically imply advancement of tumor
progression. In fact, it has been shown that older aged hosts
provide an environment which suppresses tumor growth rate and in
some cases leads to tumor regression. To assess carcinogenic risk
of extended space radiation exposure, it is important to
understand the functional dependence of response to GCR with
age. It is also of interest to identify the age window at which
humans have the lowest risk for tumor progression when exposed to
GCR.
click image to enlarge in new
window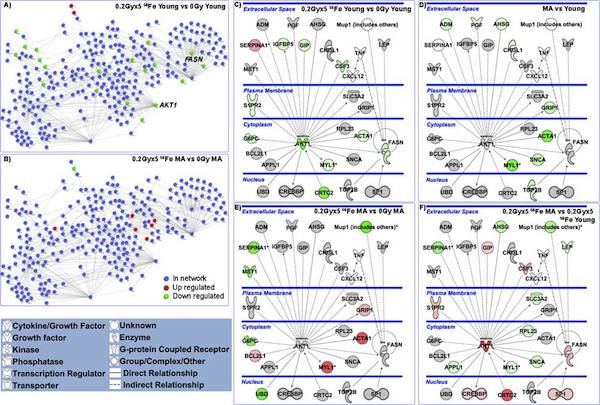 |
| Figure 3. A murine protein-protein interaction (PPI) network for signaling proteins assessed from the regulation of gene expressions. For A) & B) the network contains 281 murine proteins linked by 551 unique interactions. Blue nodes represent proteins that are in the network but are not regulated by the 56Fe irradiation perturbation, red nodes are determined to be up-regulated, and green nodes are down-regulated. A) PPI network showing regulations of tumors growing in young 56Fe-irradiated mice as compared with those in young unirradiated mice. B) PPI network regulations in tumors growing in middle-age 56Fe-irradiated mice compared with those in middle-aged unirradiated mice. For (C) – (F) pathway analysis was done with Ingenuity Pathway Analysis (IPA) software. Network depicted contains central nodes from AKT1 and FASN with direct (— solid lines) and indirect (- - dashed lines) relations to these molecules. Log2 fold changes to the gene expression were used to obtain different shades of green for regulation levels for down-regulated genes, while different shades of red depict regulation levels for up-regulated genes. Grey genes exist in the network without a significant 2-fold change under the perturbation investigated. The darker the shade of green or red, the greater the fold change. [Beheshti et al, 2013.] |
Comparing Age-Driven and Ionizing-Radiation-Driven CML (Chronic Myeloid Leukemia)
We considered SEER and atomic bomb survivor data to show that sex
differences in CML age-specific incidence are primarily due to higher
risks for males, as opposed to longer female latency periods (Fig. 4).
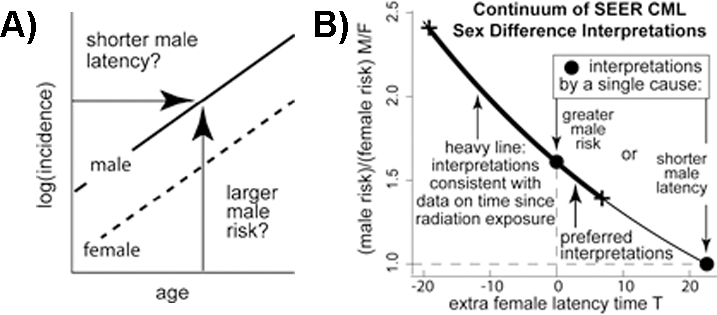 |
Figure 4. A) If CML log incidences for males and females are linear and parallel, a continuum of interpretations exists that includes: (1) males having shorter latencies between initiation and clinical CML than females but the same risks (i.e., males left of females) and (2) males having higher risks than females but no difference in latency (i.e., males above females). B) Interpretations of CML sex differences form a curve through two pure (single cause) forms (o): males with shorter latencies than females but the same risks (x-axis point) and males with higher risks than females but no difference in latency (y-axis point). Thin curve mechanisms indistinguishable by SEER data alone. Thick curve points consistent with fit to Japanese A-bomb survivor data. [Radivoyevitch et al, 2014.] |
Resources
A strong body of radiation biology work that looks at impact of age on carcinogenesis processes has been published by researchers at CCSB (click on title to go to manuscript abstract):
- Beheshti A, Wage J, McDonald JT, Lamont
C, Peluso M, Hahnfeldt P, Hlatky
L. Tumor-host signaling interaction reveals a systemic, age-dependent splenic immune influence on tumor development.
Oncotarget. 2015 Nov 3;6(34):35419-32. [Open Access]
- Wage J, Ma L, Peluso M, Lamont C,
Evens AM, Hahnfeldt P, Hlatky L, Beheshti A. Proton irradiation impacts age-driven modulations of cancer
progression influenced by immune system transcriptome modifications
from splenic tissue. J
Radiat Res. 2015 Sep;56(5):792-803. Epub 2015 Aug 7. PMCID: PMC4577010
- Beheshti A, Benzekry S, McDonald JT, Ma L, Peluso
M, Hahnfeldt P, Hlatky L. Host age is a systemic regulator of gene expression
impacting cancer progression. Cancer Res. 2015 Mar
15;75(6):1134-43. Epub 2015 Mar 2. PMCID:
PMC4397972
- Nguyen DH, Ouyang H, Mao JH, Hlatky L, Barcellos-Hoff
MH. Distinct luminal-type mammary carcinomas arise from
orthotopic Trp53-null mammary transplantation of juvenile versus
adult mice. Cancer Res. 2014 Dec 1;74(23):7149-58. Epub 2014 Oct
3. PMCID: PMC4252877 [Open Access]
- Beheshti A, Peluso M, Lamont C,
Hahnfeldt P, Hlatky L. Proton irradiation
augments the suppression of tumor progression observed with advanced
age. Radiat Res. 2014 Mar;181(3):272-83. Epub 2014 Feb
25. COVER
ARTICLE
- Radivoyevitch T, Jankovic GM, Tiu RV, Saunthararajah Y,
Jackson RC, Hlatky LR, Gale RP, Sachs RK. Sex differences in the incidence of chronic myeloid
leukemia. Radiat
Environ Biophys. 2014 Mar;53(1):55-63. Epub 2013 Dec 13. PMCID: PMC3943788
- Beheshti A, Sachs RK, Peluso M, Rietman E, Hahnfeldt P, Hlatky
L. Age and space irradiation modulate tumor progression:
implications for carcinogenesis risk. Radiat Res. 2013 Feb;179(2):208-20. Epub 2013 Jan
4.
- Radivoyevitch T, Hlatky L, Landaw J, Sachs RK. Quantitative modeling of chronic
myeloid leukemia: insights from radiobiology. Blood 2012;119(19):4363-71. Epub 2012 Feb 21. PMCID: PMC3362357 [Open Access] COVER ARTICLE
- Shuryak I, Sachs RK, Brenner DJ. Cancer risks after radiation exposure in middle age. J Natl Cancer Inst 2010;102(21):1628-36. Epub 2010 Oct 25. PMCID: PMC2970575 [Open Access]